ELISA
THE ELISA: METHOD OVERVIEW, INDIRECT ELISA, CONTRACT RESEARCH SERVICES
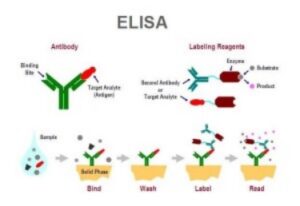
Source – Society for Mucosal Immunology
Enzyme-linked immunosorbent assays (ELISAs) are often used to measure antibody or antigen binding activity, either in a pure solution of a single protein, or in a mixed solution of multiple proteins such as serum. ELISAs perform this measurement via conversion of the binding activity of an antibody or antigen to a measurable spectrophotometric signal. ELISAs are a common assay in industrial and research applications, especially in diagnostics and quality control. This technique was first introduced in the 1970s to replace the use of radioactive isotopes in protein detection.
The ELISA technique leverages the specificity of antibodies in the detection of protein. More specifically, an enzyme is covalently linked with a target-specific antibody (either polyclonal or more reliable monoclonal antibodies). In the presence of the corresponding antigen, the antibody-enzyme is bound, any unbound antibody-enzyme complexes are removed via a washing step, and a substrate which reacts with the enzyme to produce a colored product is introduced in order to measure the antibody-antigen binding. ELISAs are typically performed in a 96-well plate and are sufficiently sensitive to detect a nanogram or less of a target antigen. Two common ELISA types used are indirect ELISA and sandwich ELISA.
INDIRECT ELISA
An indirect ELISA is utilized in the detection of antibodies. These ELISAs are performed by first coating a surface with a known amount of antigen, and then a solution with an unknown quantity of antibody (possibly patient serum) is added to the well in order to bind it to the antigen. Detection is performed with the addition of enzyme-linked antibodies (chosen due to their specificity for the antibody being measured), which are allowed to bind, and unbound antibodies are removed by washing. Substrate is added to induce coloration in the enzyme, indicating the presence of the target antibody. One application of this type of ELISA is in the test for HIV infection.
SANDWICH ELISA
Sandwich ELISAs are used to detect and quantify a particular antigen, even in small quantities. An antibody specific to the antigen being measured is coated on the bottom of a well. The solution containing the antigen is added to the well and allowed to bind to the antigen. A second, different antibody that also binds the target antigen and has an enzyme linked to it is added to the well, allowed to bind, and then excess antibody is washed off. The addition of substrate causes the presence of coloration measured by spectrophotometry, and the extent of reaction is proportional to the quantity of antigen present.
Other types of ELISA are possible to test different varieties of ligand binding or measure other biological reactions. The ELISA is a powerful tool for both industrial and research applications including diagnostics and biotechnological quality control.
OVERVIEW OF ELISA AND PLATE-BASED PROTEIN DETECTION METHODS
A key advantage to ELISAs is their use for quantification and use as a high throughput assay with ease of conversion to robotic processing. The main disadvantage is that ELISAs offer no method of antigen quality control. Therefore, certain ELISA test can be used as a rapid screening test followed by confirmatory western blots of positive samples. This is particularly important in clinical diagnostic tests.
ELISAs have the advantage of offering multiple formats compared to western blots. There are more choices available for antigen immobilization, antigen recognition and signal detection.
Antigen immobilization:
ELISAs are typically performed in 96-well plastic plates and the resulting signal detected using an automated, programmable plate reader. Antigens are first immobilized to the surface of each well and subsequent reagent additions and washes are performed in the well. Antigen immobilization can be accomplished by direct binding of antigen to the well surface.
Alternatively, antigens can be indirectly immobilized by either passive absorption to the plate surface or by specific binding through interaction antibody binding proteins. Absorbtion of a capture antibody offers the advantage of target protein enrichment leading to increased signal and reduced background. (This is similar to performing an immunoprecipitation of the target protein before a western blot analysis). In addition, immobilization of antibodies can be accomplished by either There are many options for antigen immobilization emphasizing the flexibility of the ELISA format for creative assay development.
Following immobilization, the target protein is detected either directly or indirectly using antibodies lined to enzymes, fluorophores or chromaphores for signal detection.
Direct recognition of target proteins is accomplished using primary antibodies that are directly linked to signal producing molecules. Using direct recognition can speed up the assay and reduce background but generally is less sensitive.
More commonly indirect detection is used. In this case a primary antibody that recognizes the target is applied and followed by a secondary antibody that recognizes the primary antibody through host species specific epitopes. Secondary antibodies are linked to enzymes or small molecules (i.e fluorophores) that generate a detectable signal. The use of a secondary antibody allows the advantage of signal amplification because more signal generating molecules are bound per target antigen. This will increase signal strength but can also increase background due to non-specific binding.
As with immobilization there are many detection strategies that can be used. Again, the researcher can be creative in designing the detection strategy which allows problems to solved using innovative methods. The use of fluorophores allows the option of multiplexing. Multiple targets can be simultaneously detected in one well using different secondary antibodies each conjugated to distinct fluorophores with non-overlapping emission spectra. This allows detection of multiple targets and internal normalization. This format is perfect for competitive binding studies such as epitope mapping of antibodies or competitive inhibitors of protein-protein interactions.
FOR MORE INFORMATION, SEE:
- Request quote for:
- Protein Expression Services
- Protein Expression Applications
- Assay Development
- ELISA
- Western Blot
- Stability Analysis
Laboratory Services │ Science Of Protein Expression │ Protein Expression Applications │ Assay Developmen │ ELISA │ Western Blot │ Stability Analysis │ Available Services